Highest standards of quality protection
Constant and guaranteed
bacterial strain, non-haemolytic
• Non-GMO
• Non-animal materials used during
the manufacturing process
• eye drops for dry eye (eg. artificial tears)
• intra-articular injections
• topical preparations for wound and burns healing
• intradermal injections for aesthetic correction
• anti-adhesive and shape keeping products for thoracic
and abdominal surgery
• anti-adhesive wound healing products
• bandages with exudates absorbing capacity
• Non-GMO
• Non-animal materials used during the manufacturing process
(eg. artificial tears; long molecules)
• topical preparations for wound and burns healing
Hyaluronic Acid
or Sodium Hyaluronate?
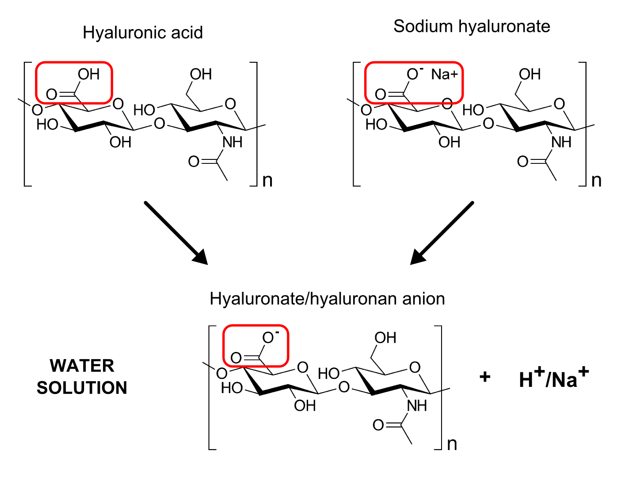
Hyaluronic acid (or hyaluronan) is a polysaccharide, which is in nature presented in a salt form, not an acid. Salt form is more stable and it is also the only form of hyaluronic acid on the market. Specifically, sodium hyaluronate is the most common one of hyaluronan salts.
Hyaluronic acid OR Sodium Hyaluronate?
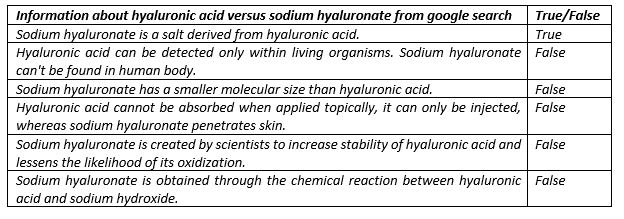
People are often confused what is the difference between hyaluronic acid (HA) and sodium hyaluronate (SHA). Indeed, if you try to google the term “hyaluronic acid vs. sodium hyaluronate”, you will find various types of articles and discussions demonstrating it is still a hot topic. We took just the first top 10 hits obtained by google search (9 of them in the field of beauty industry) and went through them. We found mistakes or misleading information in ALL of them.
Claims obtained from the first ten websites after typing search term “hyaluronic acid vs. sodium hyaluronate”:
Claim 1: Hyaluronic acid x sodium hyaluronate in nature and human body?
Hyaluronic acid, also called hyaluronan, is a polysaccharide, nonsulfated glycosaminoglycan. Nowadays, the name hyaluronan is used more prevalently because the polysaccharide is present in nature in a salt form, not an acid. The salt consists of anion hyaluronan and a cation (sodium, potassium, calcium etc.). Hyaluronic acid itself also exists, but mostly in chemical laboratories because it undergoes fast degradation and its solution is very acidic. For these reasons, under physiological conditions, you will not find hyaluronic acid in living organisms. Organic chemists sometimes use hyaluronic acid form, because it is soluble in some organic solvents which is an advantage for certain types of chemical reactions e.g. for preparation of some hyaluronic acid derivatives. However, chemists prepare hyaluronic acid by themselves from its salts e.g. by using ion exchangers which exchange sodium cations for hydrogen cations and use it immediately to prevent its degradation. Both the hyaluronic acid as well as sodium hyaluronate are soluble in water where they dissociate to the same anion so finally it is completely the same molecule you find in cosmetic products.
Claim 2: Hyaluronic acid x sodium hyaluronate on the market
Due to the stability problems and because manufacturers use organisms as a source of sodium hyaluronate (certain species of microorganisms, rooster combs, bovine vitreous humor etc.), you will not find hyaluronic acid available on the market, only its salts. The name hyaluronic acid is used as a synonym for a salt there. In cosmetic field, the INCI name Hyaluronic acid exists, but it is just a synonym for a salt as well. It refers to sodium hyaluronate which is the most common type of hyaluronic acid salt sold. Another available salts of hyaluronic acid are potassium hyaluronate with the same INCI name or calcium hyaluronate with no INCI name for instance. Sodium hyaluronate of lower molecular weights has an INCI name Hydrolyzed hyaluronic acid.
Claim 3: Sodium hyaluronate has a smaller molecular size than hyaluronic acid
There is NO difference in molecular weight between hyaluronic acid and sodium hyaluronate. You can have both molecules in a wide range of molecular weights theoretically. However, hyaluronic acid itself is not stable and easily degrades so it is complicated to prepare hyaluronic acid of high molecular weight.
Claim 4: Hyaluronic acid cannot be absorbed when applied topically, it can only be injected, whereas sodium hyaluronate penetrates skin
Hyaluronic acid is not available on the market, only salts, most commonly sodium hyaluronate. Penetration of sodium hyaluronate depends on its molecular weight. High molecular weight sodium hyaluronate is a very big molecule which does not penetrate the skin whereas the lower MW, the better penetration. A few studies are already available in the scientific literature to support this phenomenon.
Claim 5: Sodium hyaluronate is created by scientists to increase stability of hyaluronic acid and lessens the likelihood of its oxidization
This is partly true, sodium hyaluronate is indeed more stable than hyaluronic acid, but it is always produced in a form of salt by organisms so there is no need to “create” it by scientists. Oppositely, hyaluronic acid is “created” by chemists from its salts.
Claim 6: Sodium hyaluronate is obtained through the chemical reaction between hyaluronic acid and sodium hydroxide
Sodium hyaluronate is produced by animals/microorganisms as the final desired product, no further chemical reactions are needed. The product must be only isolated from the organisms using various kinds of techniques including filtrations, precipitations, washing etc.
Conclusion: hyaluronic acid vs. sodium hyaluronate
The names hyaluronic acid and sodium hyaluronate are used interchangeably as synonyms referring to sodium hyaluronate and therefore there is no difference in their physical, chemical or biological properties.